Clinical Research
Diagnostics and treatment of myeloid neoplasms in children with Down syndrome
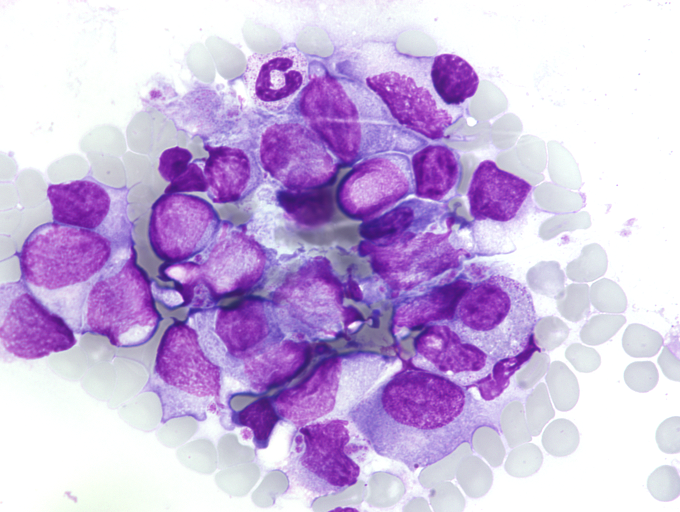
Reference laboratory
Morphology, immunophenotyping and molecular diagnostics
The ML-DS/TAM reference laboratory just moved to the Johanna Quandt Centre of the University Hospital Frankfurt. The laboratory is equipped with state-of-the-art flow cytometers (DxFLEX™ from Beckman Coulter), sorters (BD FACSAria™ Fusion) and sequencers to perform high quality morphology, immunophenotyping and molecular diagnostics. The laboratory also performs measurable residual disease (MRD) monitoring using flow cytometry and next generation sequencing, particularly for patients enrolled in the respective studies.
Children with Down syndrome (or trisomy 21) and a suspected or confirmed myeloid neoplasm can submit material (blood and blood smears, bone marrow aspirates, CSF) to our laboratory for a reference opinion. When submitting material, please download and use the submission form.
ML-DS 2018 trial
Phase III clinical trial for CPX-351 in myeloid leukemia in children with Down syndrome 2018
Prof. Dr. Jan-Henning Klusmann is the International Coordinating Investigator of the Phase III Clinical Trial for CPX-351 in Myeloid Leukemia in Children with Down Syndrome 2018 – a prospective, non-randomized, open-label international, and multicenter phase III trial for children with myeloid leukemia associated with Down syndrome (ML-DS). The trial aims to investigate whether substituting the induction of standard ML-DS therapy (as defined in the ML-DS 2006 protocol) with CPX-351, and reducing treatment intensity in course 4 in patients with a good response, can reduce toxicity while maintaining excellent patient outcomes. The reduction of treatment-related toxicity is particularly important for children with Down syndrome, who are very vulnerable to the side effects of intense chemotherapy.
Therapy recommendations
Recommendations for the diagnosis and treatment of children with transient abnormal myelopoiesis (TAM) and myeloid leukemia in Down syndrome (ML-DS)
Children with Down syndrome are at a high risk of developing transient abnormal myelopoiesis (TAM, or TMD) or myeloid leukemia (ML-DS). While most patients with TAM are asymptomatic and go into spontaneous remission without needing therapy, TAM-related complications can be fatal and many patients progress from TAM to ML-DS. ML-DS patients are particularly vulnerable to therapy-associated toxicity, but the prognosis of relapsed ML-DS is extremely poor – thus, ML-DS therapy schemata must strive for a balance between appropriate efficacy (to avoid relapses) and treatment-related toxicity.
We recently compiled diagnostic and therapeutic guidelines for TAM and ML-DS based on the experience and results of previous clinical studies from the Berlin-Frankfurt-Münster (BFM) working group. These protocols have helped reduce the risk of early death in symptomatic TAM patients using low-dose cytarabine, and achieved excellent cure rates for ML-DS using intensity-reduced treatment protocols.